
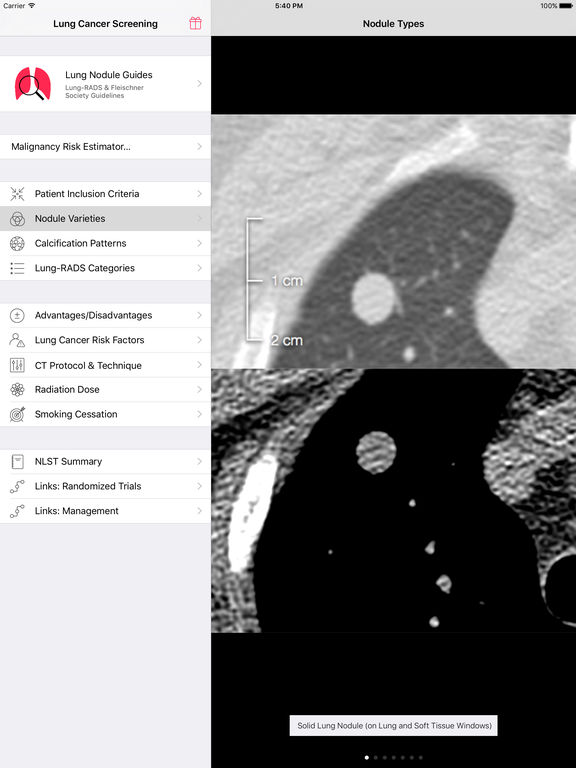
1.3.10.Īudit the local test performance of EBUS-TBNA and endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA).
1.3.9.Įvery cancer alliance should have at least 1 centre with EBUS and/or endoscopic ultrasound (EUS) to ensure timely access. Offer endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) for biopsy of paratracheal and peri-bronchial intra-parenchymal lung lesions. Use MRI when necessary to assess the extent of disease, for people with superior sulcus tumours. 1.3.6.ĭo not routinely use MRI to assess the stage of the primary tumour (T-stage) in non-small-cell lung cancer (NSCLC). 1.3.5.Įvery cancer alliance should have a system of rapid access to PET-CT scanning for eligible people. Įnsure that all people with lung cancer who could potentially have treatment with curative intent are offered positron-emission tomography CT (PET-CT) before treatment. Be aware that surgical assessment may be necessary if there are no contraindications to resection.


 0 kommentar(er)
0 kommentar(er)
